Sublingual Administration of Oral Estradiol Valerate Tablets for Transfeminine People
By Aly | First published January 5, 2019 | Last modified November 28, 2022
Abstract / TL;DR
Oral estradiol tablets can be taken sublingually instead of orally and this allows for greater bioavailability and higher estradiol levels than with oral use. For this reason, sublingual estradiol is frequently used in transfeminine people. Oral estradiol valerate tablets also exist in some parts of the world and it’s often inquired by transfeminine people whether this form of estradiol is likewise effective sublingually. Research in this area is limited and no direct comparisons exist, but at least one study in cisgender women has shown that oral estradiol valerate tablets taken sublingually can result in high estradiol levels and gonadal suppression analogously to sublingual estradiol. In addition, at least one gender-affirming hormone therapy clinic has reported use of sublingual estradiol valerate in transfeminine people. Hence, sublingual estradiol valerate appears to be an effective means of estradiol delivery similarly to sublingual estradiol. One difference between these forms of estradiol is that estradiol valerate contains less estradiol than estradiol by weight and hence should be taken at slightly higher doses. While they can be effective, oral estradiol tablets and oral estradiol valerate tablets were not intended or designed for sublingual use, and formulations of these tablets vary. The implications of this in terms of clinical properties, if any, are unknown. However, it does seem apparent that different tablet formulations may require substantially different amounts of time to dissolve when used sublingually. As a result, some formulations of oral estradiol and oral estradiol valerate may be better-suited for sublingual use than others. Additional characteristics of these formulations like micronization and lipophilicity could also differentially influence their pharmacokinetics in ways that have not been studied. It may be advisable to choose sublingual estradiol over sublingual estradiol valerate where possible simply because sublingual estradiol is much better-characterized in comparison and there are fewer unknowns with it. However, sublingual estradiol valerate can be a clearly effective form of estradiol for transfeminine people as well if needed.
Introduction
Oral estradiol tablets (e.g., Estrace and Estrofem among other brand names) are indicated for oral administration (i.e., taken by mouth/swallowed) and this is how they are normally taken. As an alternative to the standard oral route however, these tablets can be taken sublingually (held under the tongue) or buccally (held in the cheek or lips/gums). Sublingual or buccal administration of oral estradiol tablets allows for much greater bioavailability and estradiol levels in comparison to oral administration (Sam, 2021; Wiki; Graphs; Wiki). Transfeminine people often use sublingual estradiol as the estrogen component of hormone therapy. In various countries, for instance many European countries, estradiol is also provided in oral form as estradiol valerate (EV) tablets (e.g., Progynova among other brand names). It is frequently inquired by transfeminine people whether estradiol valerate tablets can be taken sublingually similarly to estradiol tablets and whether there are any differences between these two estradiol forms for this route. This article is intended to explore and shed some light on these questions.
Effectiveness of Sublingual Estradiol Valerate
Estradiol valerate is an estradiol ester and a prodrug of estradiol. Estradiol esters themselves are pharmacologically inactive prior to conversion into estradiol. Estradiol valerate and other related estradiol esters are cleaved into estradiol by various esterase enzymes. These esterases are widely expressed throughout the body, and the metabolism of estradiol esters into estradiol occurs not only in the liver but has also been shown to take place rapidly in blood and other tissues (Wiki). Hence, estradiol esters like estradiol valerate do not require the first pass through the liver that occurs with oral administration to become pharmacologically active as estrogens. As such, transformation of estradiol valerate into estradiol should not be an impediment in terms of non-oral administration of estradiol valerate, for instance via sublingual administration (as well as in the form of depot injectables of course).
Studies of estradiol tablets administered sublingually instead of orally are limited, and studies on estradiol valerate tablets used sublingually are extremely scarce. No direct comparisons have been made between sublingual use of estradiol tablets versus sublingual use of estradiol valerate tablets. Hence, we currently don’t have reliable data on how sublingual estradiol valerate compares to sublingual estradiol in terms of pharmacokinetics (e.g., bioavailability, estradiol levels, concentration–time curve, etc.).
Only one study seems to have researched and characterized sublingual administration of oral estradiol valerate tablets. This study assessed the use of oral estradiol valerate tablets (brand name Progynova [Schering]) administered sublingually 3 to 4 times per day in a group of premenopausal cisgender women. The researchers reported the findings of their study in the following two publications:
- Serhal, P., & Craft, I. (1989). Oocyte donation in 61 patients. The Lancet, 333(8648), 1185–1187. [DOI:10.1016/S0140-6736(89)92762-1]
- Serhal, P. (1990). Oocyte donation and surrogacy. British Medical Bulletin, 46(3), 796–812. [DOI:10.1093/oxfordjournals.bmb.a072432]
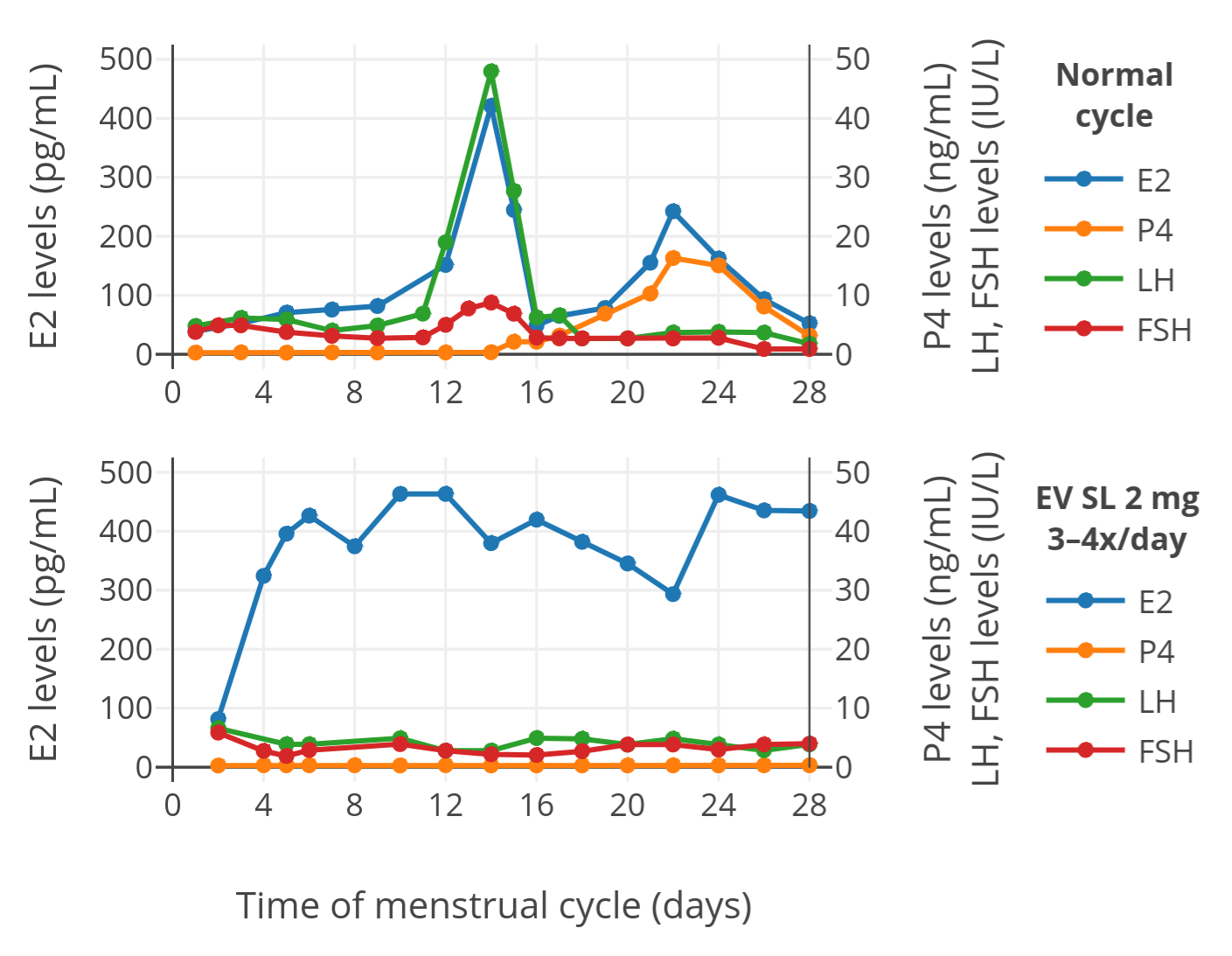
The results in terms of hormone levels with sublingual estradiol valerate were as follows (top plot is a control menstrual cycle in untreated women, bottom plot is a cycle with sublingual estradiol valerate):
 |
|---|
| Figure 1: Levels of estradiol (E2), progesterone (P4), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) with 2 mg oral micronized estradiol valerate tablets (Progynova) administered sublingually (SL) 3 or 4 times per day in a group of premenopausal women (bottom). The normal menstrual cycle in a control group of premenopausal women is also shown (top). The time of blood collection following administration was not specified. |
As can be seen in the figure, estradiol levels with sublingual estradiol valerate were much higher than in normally cycling control women. In addition, levels of other hormones were suppressed, which is in accordance with the high estradiol levels exhibiting negative feedback on the hypothalamic–pituitary–gonadal axis and suppressing hormone production. These findings indicate that sublingual estradiol valerate is well-absorbed and is able to achieve high estradiol levels analogously to sublingual estradiol. In other words, although we still don’t have direct comparisons between the two, sublingual estradiol valerate appears to be a highly effective means of estradiol delivery similarly to sublingual estradiol.
It’s notable that at least one gender-affirming hormone therapy clinic, located in South Korea, uses sublingual estradiol valerate in transfeminine people. These clinicians have briefly described their experience with sublingual estradiol valerate and their rationale for using it over oral estradiol in a recent publication (Lim et al., 2019). While they don’t provide actual pharmacokinetic data on sublingual estradiol valerate (e.g., estradiol levels), it seems apparent based on their clinical experience that sublingual use is a therapeutically effective route of administration for this form of estradiol.
Formulation of Estradiol and Estradiol Valerate Tablets and Implications for Sublingual Use
Micronization of Oral Estradiol Valerate Tablets
Micronization is a manufacturing process in which solid particles of a substance are reduced to smaller sizes. Micronization can modify the pharmacokinetics of medications by altering their rate and extent of absorption. Oral estradiol tablets were originally non-micronized and had lower estrogenic potency than those used today, but in the 1970s micronized oral estradiol tablets were introduced and replaced the previous formulations (Wiki; Wiki). It appears that micronization of estradiol crystals to a defined particle size range improves the absorption and bioavailability of oral estradiol tablets by several-fold (Wiki). All modern oral estradiol tablets available today are assumed to be micronized.
Questions arise as to what influence micronization has on sublingual administration (rather than oral administration) of hormonal agents like estradiol and estradiol valerate and whether oral estradiol valerate tablets are micronized similarly to oral estradiol tablets. The influence of micronization on the sublingual absorption and pharmacokinetic characteristics of estradiol and its esters, as well as other hormonal agents like progesterone and testosterone, does not seem to have been studied and hence is unknown. It could be assumed that micronization might improve the rate and extent of absorption with sublingual administration similarly to oral administration and hence may be important however, as touched on by the following literature excerpt (Sayeed & Ashraf, 2014):
The drugs that are administered sublingually generally have low solubility. Therefore, to enhance dissolution, it is crucial to reduce and control the particle size of the [active pharmaceutical ingredient]. This attribute is important in the case of all drugs with low solubility. However, a tighter control on particle size of [active pharmaceutical ingredient] is desirable in sublingual drug products to maintain the reproducible quality and performance of the drug product in view of the limited window of dissolution and absorption time.
As to whether oral estradiol valerate tablets are micronized, some formulations of oral estradiol valerate clearly indicate that they are micronized in their packaging or manufacturer information (Photo), whereas other formulations of oral estradiol valerate do not. The oral estradiol valerate tablets used in the study by Serhal and Craft (Progynova [Schering]) as well as certain other publications (e.g., Devroey & Pados, 1998) have been explicitly noted to be micronized. Considering the similar doses and apparently comparable clinical properties of all oral estradiol valerate tablets used today, it may be the case that all oral estradiol valerate formulations are micronized but that this simply isn’t always explicitly stated. Indeed, the original form of oral estradiol valerate introduced in the late 1960s is said to have been micronized (Wiki). An alternative but perhaps less likely possibility is that micronization might not influence the properties of oral estradiol valerate similarly to how it does with oral estradiol.
The University of California, San Francisco (UCSF) transgender care guidelines state that only micronized oral estradiol tablets can be used sublingually and imply that not all oral estradiol tablets are micronized (Deutsch, 2016). These statements may be assumed to also apply to oral estradiol valerate tablets. However, as discussed in this article, support for these notions is lacking at present.
Physicochemical Properties of Estradiol versus Estradiol Valerate
Estradiol valerate is more lipophilic (fat-soluble) than estradiol due to its fatty acid ester moiety (i.e., valeric acid). Lipophilicity is known to modify the sublingual and buccal absorption of medications (Smart, 2005; Batheja, Thakur, & Michniak, 2006). How the differing lipophilicities of estradiol versus estradiol valerate may influence their pharmacokinetics when used sublingually has not been studied. Hence, the therapeutic implications of this physicochemical difference for this route are unknown. It’s also known however that esterases are present in the oral mucosa and saliva and can cleave carboxylic acid esters like estradiol valerate into their unesterified forms (Yamahara & Lee, 1993; Rathbone, Drummond, & Tucker, 1994). This might serve to reduce the importance of the physicochemical differences between estradiol valerate and estradiol in terms of sublingual and buccal administration.
Another difference between estradiol and estradiol valerate is that estradiol valerate has a higher molecular weight than estradiol due to its ester component and hence estradiol valerate contains less estradiol than estradiol for the same dose of substance. The molecular weight of estradiol valerate is about 131% of that of estradiol and hence estradiol valerate contains 76% of the estradiol as an equal amount of estradiol (Table). This difference has been shown to translate to pharmacokinetic studies of oral estradiol versus oral estradiol valerate, with estradiol levels being around 25% lower with oral estradiol valerate compared to oral estradiol at equal doses (Wiki). This is likely also the case for other routes of administration of these forms of estradiol, including sublingual administration. Hence, slightly higher doses (e.g., 2 mg versus 1.5 mg) are likely needed and should be used for estradiol valerate relative to estradiol for equivalent estradiol levels and therapeutic estrogenic effect (Sam, 2021).
Formulation and Dissolution of Oral Estradiol Valerate Tablets
Oral estradiol and estradiol valerate tablets were intended and designed for oral administration and not specifically for sublingual administration. Although many of these tablets do clearly work quite well when used sublingually, different tablet formulations vary in their coating and their compositions and excipients. It is possible that differences between formulations of these tablets may influence their properties when used sublingually, as touched on in the following literature excerpt (Sayeed & Ashraf, 2014):
The conditions prevailing in the oral cavity for disintegration and dissolution of sublingual tablets are markedly different from the tablets that are orally ingested. […] Other specialized [oral] tablets, such as modified-release or enteric-coated tablets, may also partly release the drug in the stomach. In contrast, sublingual tablets are designed to completely disintegrate and dissolve in the oral cavity under the tongue.
One particular issue is that different oral tablets of estradiol and estradiol valerate may dissolve at very different rates. One study of oral estradiol tablets used sublingually reported that they dissolved within 1 or 2 minutes (Burnier, 1981). Anecdotally this has been the case similarly with one brand of generic sugar-coated oral estradiol tablets in the United States (Photo), which dissolve and disappear sublingually within a few minutes at most. Some transfeminine people on social media sites like Reddit however have reported their tablets taking much longer to dissolve, for instance about an hour, when used sublingually or buccally. In any case, dissolution rates of oral estradiol and estradiol valerate tablets may vary depending on the formulation, and some forms of oral estradiol and oral estradiol valerate may be better-suited for sublingual use than others. If tablets take a long time to dissolve when used sublingually, switching to another brand may be considered.
Estradiol or Estradiol Valerate for Sublingual Use?
Sublingual administration of oral estradiol tablets has been much better researched and characterized than sublingual administration of oral estradiol valerate tablets. Because of this, it may be advisable to select oral estradiol tablets with the intention of sublingual use over oral estradiol valerate tablets simply because there are fewer unknowns with them. However, oral estradiol tablets may not always be available in a given market, a person may be prescribed oral estradiol valerate tablets instead of oral estradiol tablets, or other considerations may make oral estradiol tablets a less feasible option. In this regard, it is clear based on available literature that sublingual estradiol valerate can be a highly effective means of delivering estradiol similarly to sublingual estradiol and can be used instead if needed.
References
- Batheja, P., Thakur, R., & Michniak, B. (2006). Basic Biopharmaceutics of Buccal and Sublingual Absorption. In Touitou, E., & Barry, B. W. (Eds.). Enhancement in Drug Delivery (pp. 175–202). Boca Raton/London/New York: CRC Press. [Google Scholar] [Google Books] [DOI:10.1201/9781420004816-17]
- Burnier, A. M., Martin, P. L., Yen, S. S., & Brooks, P. (1981). Sublingual absorption of micronized 17β-estradiol. American Journal of Obstetrics and Gynecology, 140(2), 146–150. [DOI:10.1016/0002-9378(81)90101-0]
- Deutsch, M. B. (2016). Overview of feminizing hormone therapy. In Deutsch, M. B. (Ed.). Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People, 2nd Edition (pp. 26–48). San Francisco: University of California, San Francisco/UCSF Transgender Care. [Google Scholar] [URL] [PDF]
- Devroey, P., & Pados, G. (1998). Preparation of endometrium for egg donation. Human Reproduction Update, 4(6), 856–861. [DOI:10.1093/humupd/4.6.856]
- Lim, H. H., Jang, Y. H., Choi, G. Y., Lee, J. J., & Lee, E. S. (2019). Gender affirmative care of transgender people: a single center’s experience in Korea. Obstetrics & Gynecology Science, 62(1), 46–55. [DOI:10.5468/ogs.2019.62.1.46]
- Rathbone, M. J., Drummond, B. K., & Tucker, I. G. (1994). The oral cavity as a site for systemic drug delivery. Advanced Drug Delivery Reviews, 13(1–2), 1–22. [DOI:10.1016/0169-409X(94)90024-8]
- Sam. (2021). An Exploration of Sublingual Estradiol as an Alternative to Oral Estradiol in Transfeminine People. Transfeminine Science. [URL]
- Sayeed, V. A., & Ashraf, M. (2014). Considerations in Developing Sublingual Tablets—An Overview. Pharmaceutical Technology, 38(11), 34–72. [Google Scholar] [URL] [PDF]
- Serhal, P., & Craft, I. (1989). Oocyte donation in 61 patients. The Lancet, 333(8648), 1185–1187. [DOI:10.1016/S0140-6736(89)92762-1]
- Serhal, P. (1990). Oocyte donation and surrogacy. British Medical Bulletin, 46(3), 796–812. [DOI:10.1093/oxfordjournals.bmb.a072432]
- Smart, J. D. (2005). Buccal drug delivery. Expert Opinion on Drug Delivery, 2(3), 507–517. [DOI:10.1517/17425247.2.3.507]
- Yamahara, H., & Lee, V. H. (1993). Drug metabolism in the oral cavity. Advanced Drug Delivery Reviews, 12(1–2), 25–39. [DOI:10.1016/0169-409X(93)90039-7]